Introduction to Diagnostic Medical Parasitology
Trypanosomiasis, african
Essentials
The haemoflagellates Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense are the causal agents of African sleeping sickness. T. brucei causes the disease nagana in cattle.
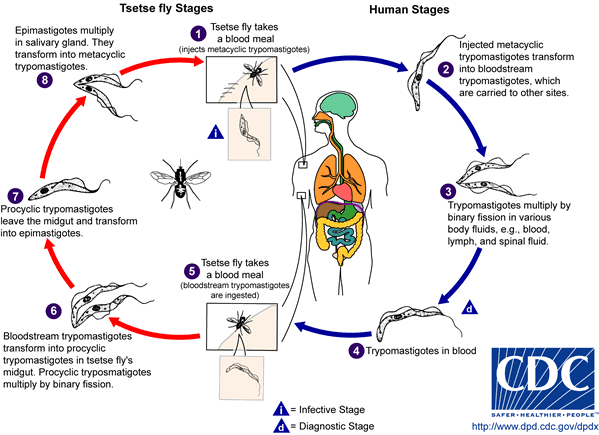
Infection is by the bite of an infectious tsetse fly (Glossina spp.). The tsetse fly is infected by ingesting blood containing trypanosomes from an infected human or animal. The parasite multiplies in the tsetse fly for 12-30 days. Finally, infective forms develop in the salivary glands. Once infected, the tsetse fly remains infected for life (3-10 months)!

Epidemiology
- African trypanosomiasis – sleeping sickness – is confined to tropical Africa between latitudes 15ºN and 20ºS (the “tsetse belt”)
- T.b. gambiense occurs in Saharan savannah and riverine forests of western and central Africa
- T.b. rhodesiense occurs in the savannah of eastern and south-east Africa
- The pathogen is transmitted by blood-sucking tsetse flies
- Wild animals (antelopes, lions) are carriers of T. brucei rhodesiense
- Domestic and wild animals are reservoirs for T. brucei gambiense



Pathology
- One week after the chancre appears, the infection reaches the regional lymph nodes, causing lymphadenitis. Puncture of these lymph nodes will reveal trypanosomes.
- In the late stage, there is body wasting, somnolence and signs showing involvement of the CNS (reversal of sleeping pattern)
- Disease due to T. brucei gambiense may run a protracted course of several years
- Disease due to T. brucei rhodesiense is lethal within weeks or a few months without treatment. CNS invasion and symptoms are seen within weeks.
Clinical Findings
- In the early stage following a tsetse fly bite, a papule forms, which evolves into a nodule (mainly T. brucei rhodesiense) and finally into a painful chancre.
- In the haematolymphatic stage, many symptoms (fever, headaches, joint pains and malaise) recur at regular intervals with the observed parasitaemia peaks
- In the meningoencephalitic stage, there are sign and symptoms due to brain lesions (disturbed sleep patterns, insomnia, anorexia, nerve dysfunctions)

Diagnosis
Diagnostic methods
Parasitological diagnosis
Diagnosis may be achieved by microscopic detection of trypanosomes in blood, in chancre aspirates, in lymph or cerebrospinal fluids. However, sensitivity is limited even when parasite concentration techniques (micro-haematocrit or the mini-anion exchange centrifugation technique) are used. Sensitivity is less a problem in acute T.b. rhodesiense infections, where trypanosomes are numerous. Multiple specimens should be collected because parasite numbers fluctuate!
Caution when handling specimens of infected patients, which are highly infectious!
Molecular diagnosis
Nested PCR tests have been developed to differentiate trypanosome species in domestic and wild animals for epidemiological studies. A recent paper describes a multiplex PCR that discriminates between T.b. brucei and zoonotic T.b. rhodesiense.
Antigen detection
An ELISA method is available to detect antigen in serum and cerebrospinal fluid. A simple rapid test, the card indirect agglutination test (CIATT), is available for field surveys.
Antibody detection
Specific antibodies may be demonstrated by ELISA, IFAT and the card agglutination test (CATT). Serology is important in chronic sleeping sickness due to T.b. gambiense.
For serological screening of T. b. gambiense, the feasibility to detect antibodies in human saliva has been proven.
High levels of mainly non-specific IgM are common in African trypanosomiasis due to polyclonal activation of lymphocytes.
Diagnostic strategies
- For screening a population in an endemic area
Rapid antigen detection tests are most convenient to assess endemicity.
- For diagnosing an individual case
As a first attempt, one tries to demonstrate trypanosomes in the blood directly or by using concentration methods. As additional methods, antigen – and antibody – detection could be used if blood examinations are negative.
- For evaluating control measures
Be aware that in view of the potentially low prevalence after an intervention, you have to select a diagnostic strategy assuring a high predictive value for positive results. This means you should choose a single method (or eventually a combination of methods) with a high specificity!
To get an exact value for the relative risk in a placebo-controlled intervention study (e.g. a vaccine trial), the specificity should ideally be 100%.
Prevention and control
- Educate the public about protective measures against tsetse fly bites
- Control of the vector (by fly traps or insecticides)
- New epidemics occur when control programmes break down