Introduction to Diagnostic Medical Parasitology
Essentials
Schistosomiasis or bilharziasis is a significant parasitic disease in the tropics, present in over 70 countries. S. mansoni, S. haematobium (found only in Africa) and S. japonicum (found only in Asia) are of major importance. Two further species (S. intercalatum in central Africa and S. mekongi in South-East Asia) are more localised. The World Health Organisation (WHO) estimates that, today, around 600 million people are exposed to and 200 million people are infected with schistosomes. Urinary schistosomiasis is due to S. haematobium. Adult male and female flukes (up to 2 mm in length) live as pairs in the vesical and pelvic plexus.
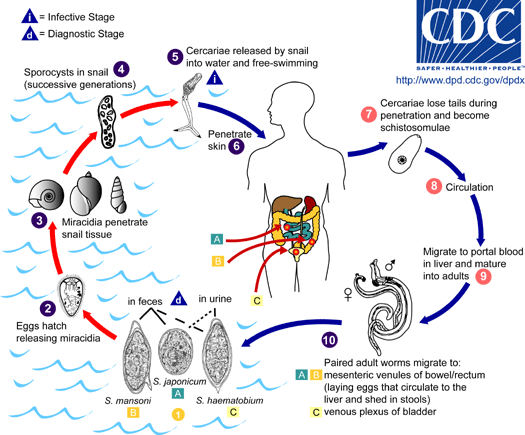
The transmission of urinary schistosomiasis from one patient to another can only take place indirectly. Eggs with a fully developed embryo (miracidium) are passed with the faeces and hatch when in contact with water. Miracidia must enter a freshwater snail (of the genus Bulinus) for further development and asexual proliferation. The resulting forked-tailed cercariae are infectious for humans. They attach to the skin and bury themselves actively into it, shedding the forked tail. The maturation of cercariae to adult parasites involves a complex migration through the body and additional development. In the skin, the tailless cercaria becomes a schistosomulum. Via lymph vessels or the venous blood circulation through the heart, schistosomulae reach the lungs in approximately 4-10 days. They migrate further against the blood flow into the liver. The pairing of the sexed worms (an exception among the generally hermaphroditic trematodes!) leads to sexual maturation. Paired worms migrate to the vesical and pelvic plexus. Adult worms have an average life expectancy of 6-10 years. The deposition of eggs begins 5-6 weeks post-infection. Most eggs are found in the urine but some eggs might also be found in the faeces.
Epidemiology
- S. haematobium is focally distributed in Africa and the Middle East (prevalence is especially high in West Africa and the Nile Valley)
- Humans are infected by penetrating cercariae
- Schoolchildren have the highest worm burden in endemic areas. A partial immunity (“concomitant immunity”) protects adults from reinfection.
- Building of dams and irrigation system favours habitats for the intermediate host snail

Pathology
- Schistosomiasis is mainly an “immunological disease”
In early infections one might observe:
- Dermatitis (a reaction against invading cercariae)
- Katayama fever (an acute syndrome against schistosomulae in the lungs)
In chronic infections:
- Granuloma formation around eggs deposited in tissues (mainly the liver) leading to hepatic portal fibrosis, portal hypertension and hepatosplenomegaly in heavy infections - Damage is also induced mechanically by eggs which migrate from blood vessels to the vesicular lumen (leading to haematuria)
Clinical Findings
- Signs and symptoms of acute schistosomiasis (Katayama fever) are rather rare for S. haematobium infections
- In chronic infections (correlated with the worm load), damage is mainly seen in the bladder and the ureter. Signs and symptoms are urethral pain, dysuria, terminal haematuria, proteinuria
- Severe complications in a small proportion of cases manifest as hepatosplenomegaly or portal-systemic collateral circulation (eggs bypass the liver and damage the lungs or the heart)

Diagnosis
Diagnostic methods
Parasitological diagnosis
Detection of ova in urine samples using sedimentation or filtration methods has limited sensitivity in light or chronic infections. Even in heavy infections, false-negative urine results can be found since egg excretion varies considerably between days. Several samples (3–5) have to be analysed to securely exclude an infection.
Molecular diagnosis
First attempts have been described to detect parasite DNA in stool samples by PCR. However this method is not yet fully validated.
As a research tool, molecular techniques, such as restriction fragment length polymorphism (RFLP) analysis, polymerase chain reaction (PCR) and DNA sequencing are now proving invaluable for distinguishing species and strains of schistosomes.
Antigen detection
First attempts to detect circulating proteoglycans in blood or urine of infected patients by various immunological methods started over 30 years ago. Today a reagent strip test for the detection of the circulating cathodic antigen (CCA) in the urine is close to an end-user format.
Antibody detection
There are many home-made serological assays developed by different laboratories. The most frequently used test formats are the indirect fluorescent antibody test (IFA) using frozen sections of adult worms, and immunoenzyme assays (mostly ELISAs) with crude or recombinant antigens from adult worms or eggs.
Diagnostic strategies
- To assess the prevalence of schistosomiasis in an endemic area
For rapid assessment, questionnaires can be used (e.g. in schools) and validated (in a smaller sample) by urine filtrations
- To confirm a symptomatic case outside endemic areas
A combination of serology and one or several urine examinations seems the best available diagnostic strategy today. If an indicator case is observed in an exposed group of people (e.g. in a family), all (also asymptomatic) members should be screened by serology.
- To assess drug therapy in a returning traveller
Multiple urine samples would be the ideal approach to monitor drug therapy. In cases where a blood hypereosinophilia is detected before treatment, one might also monitor the number of eosinophils.

Prevention and control
- Health education in endemic areas (especially in schools) is very important
- The life cycle can be interrupted at various points:
- by providing sanitary installations (to prevent the infection of snails)
- by eliminating snails with molluscicides (rarely effective!)
- by preventing human contacts with cercariae (safe water supply, preventing access to transmission sites etc.)