Introduction to Diagnostic Medical Parasitology
Schistosomiasis, intestinal
Essentials
Schistosomiasis or bilharziasis is a significant parasitic disease in the tropics, present in over 70 countries. S. mansoni, S. haematobium (found only in Africa) and S. japonicum (found only in Asia) are of major importance. Two further species (S. intercalatum in central Africa and S. mekongi in South-East Asia) are more localised. The World Health Organisation (WHO) estimates that, today, around 600 million people are exposed to and 200 million people are infected with schistosomes. Intestinal schistosomiasis is due to S. mansoni, S. japonicum or S. mekongi. Adult male and female flukes (up to 2 mm in length) live as pairs in the mesenteric veins.
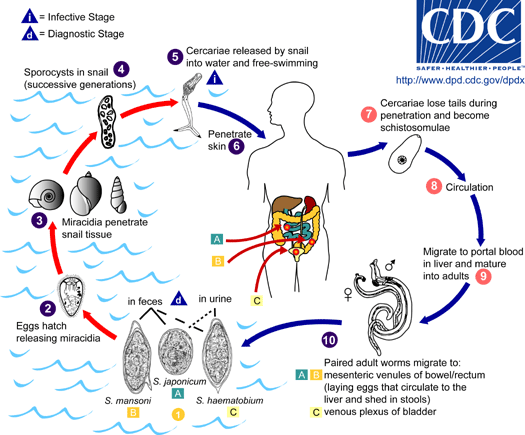
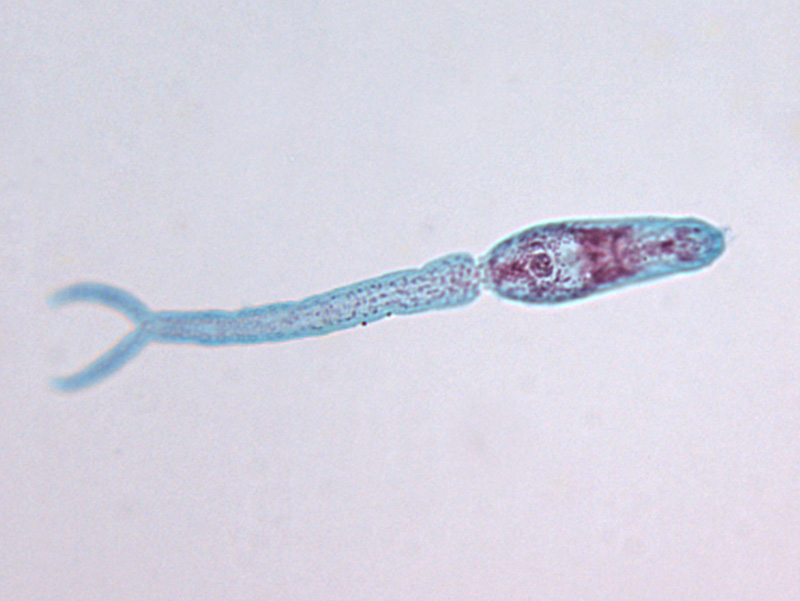
The transmission of intestinal schistosomiasis from one patient to another can only take place indirectly. Eggs with a fully developed embryo (miracidium) are passed with the faeces and hatch when in contact with water. Miracidia must enter a freshwater snail (Biomphalaria spp. for S. mansoni, Oncomelania spp. for S. japonicum, Neotricula spp. for S. mekongi) for further development and asexual proliferation. The resulting forked-tailed cercariae are infectious for humans. They attach to the skin and bury themselves actively into it, shedding the forked tail. The maturation of cercariae to adult parasites involves a complex migration through the body and additional development. In the skin, the tailless cercaria becomes a schistosomulum. Via lymph vessels or the venous blood circulation through the heart, schistosomulae reach the lungs in approximately 4-10 days. They migrate further against the blood flow into the liver. The pairing of the sexed worms (an exception among the generally hermaphroditic trematodes!) leads to sexual maturation. Paired worms migrate into the mesenteric veins. Adult worms have an average life expectancy of 6-10 years. The deposition of eggs begins 5-6 weeks post-infection.



Epidemiology
- S. mansoni is endemic in Africa (including Madagascar), the Arabian peninsula and parts of South America
- S. japonicum is distributed in China, Taiwan, the Philippines and Sulawesi (Celebes)
- S. mekongi occurs in the Mekong River area of Laos, Cambodia and Thailand
- Humans become infected when they have contact (during bathing, washing, fishing) with freshwater into which snails have shed cercariae
- Schoolchildren have the highest worm burden in endemic areas. A partial immunity (“concomitant immunity”) protects adults from reinfection
- Building of dams and irrigation system favours habitats for the intermediate host snail


Pathology
- Schistosomiasis is mainly an “immunological disease”
In early infections one might observe:
- Dermatitis (a reaction against invading cercariae)
- Katayama fever (an acute syndrome against schistosomulae in the lungs)
In chronic infections:
- Granuloma formation around eggs deposited in tissues (mainly the liver) leading to hepatic portal fibrosis, portal hypertension and hepatosplenomegaly in heavy infections - Damage is also induced mechanically by eggs which migrate from blood vessels to the intestinal lumen (intestinal haemorrhage)
Clinical Findings
- Signs and symptoms during acute schistosomiasis (Katayama fever) which manifests only in hyper-responsive patients are: fever, chills, dry cough, anorexia, nausea, vomiting, high blood eosinophilia
- In chronic infections one might detect abdominal pain, blood in stools, portal hypertension
- Severe complications in a small proportion of cases are hepatosplenomegaly, portal-systemic collateral circulation (eggs bypass the liver and damage the lungs or the heart), colonic polyposis
Diagnosis
Diagnostic methods
Parasitological diagnosis
Detection of ova in stool samples has limited sensitivity even in heavy infections since egg excretion varies considerably between days. Several samples (3–5) have to be analysed to achieve a high predictive value for a negative result.
Molecular diagnosis
First attempts have been described to detect parasite DNA in stool samples by PCR. This method is not yet fully validated.
As a research tool, molecular techniques, such as restriction fragment length polymorphism (RFLP) analysis, polymerase chain reaction (PCR) and DNA sequencing are now proving invaluable tools for distinguishing species and strains of schistosomes.
Antigen detection
First attempts to detect circulating proteoglycans in blood or urine of infected patients by various immunological methods started over 30 years ago. Today, a reagent strip test for the detection of the circulating cathodic antigen (CCA) in the urine is close to an end-user format.
Antibody detection
There are many home-made serological assays developed by different laboratories. The most frequently used test formats are the indirect fluorescent antibody test (IFA) using frozen sections of adult worms, and immunoenzyme assays (mostly ELISAs) with crude or recombinant antigens from eggs or adult worms.
Diagnostic strategies
- To assess the prevalence of schistosomiasis in an endemic area
For rapid assessment, questionnaires (e.g. in schools) can be used and validated (in a smaller sample) by Kato-Katz stool examinations
- To confirm a symptomatic case outside endemic areas
A combination of serology and one or several stool examinations seems the best available diagnostic strategy today. If an indicator case is observed in an exposed group of people (e.g. in a family), all (also asymptomatic) members should be screened by serology.
- To assess drug therapy in a returning traveller
Multiple stool samples would be the ideal approach to monitor drug therapy. In cases where a blood hypereosinophilia is detected before treatment, one might also monitor the number of eosinophilic granulocytes.



Prevention and control
- Health education in endemic areas (especially in schools) is very important
- The life cycle can be interrupted at various points:
- by providing sanitary installations (to prevent infection of snails)
- by eliminating snails with molluscicides (rarely effective!)
- by preventing human contacts with cercariae (safe water supply, preventing access to transmission sites etc.)
- by mass treatment in endemic areas (to reduce egg excretion and infection of intermediate hosts)
